Featured
- Get link
- X
- Other Apps
What Type Of Chemical Reaction Connects Amino Acids
What Type Of Chemical Reaction Connects Amino Acids. The acid group becomes negative, and the amine nitrogen becomes positive because of the. Ninhydrin is used to detect fingerprints because it reacts with amino acids from the.
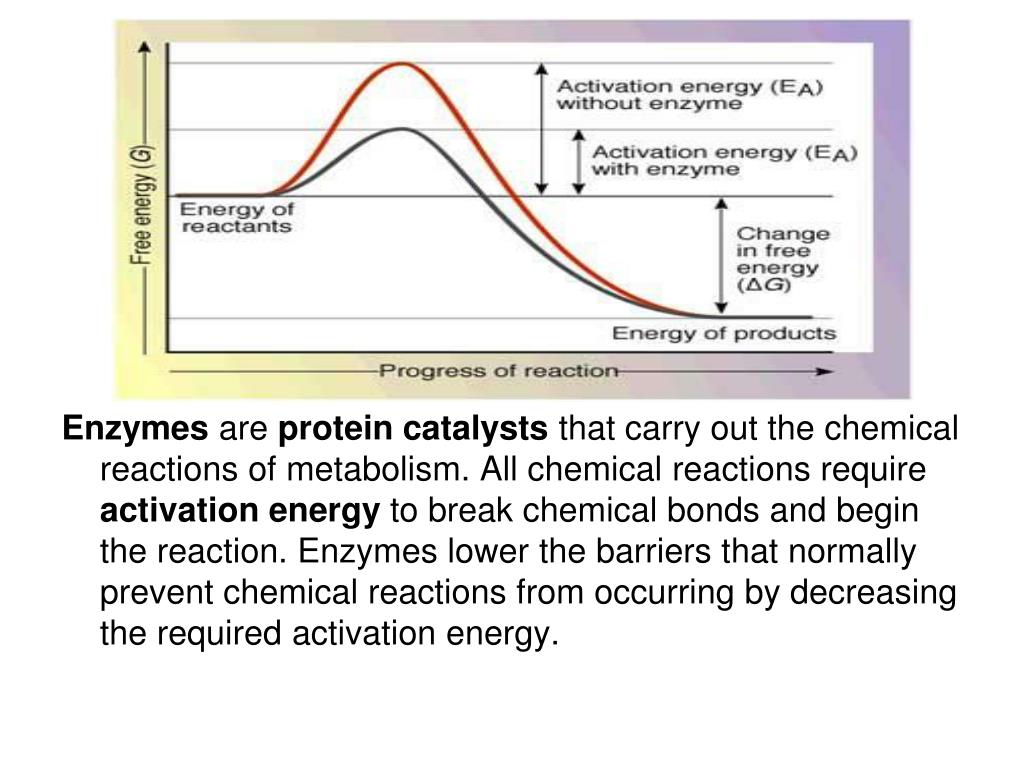
Chemical reactions of amines 1) reaction with nitrous acid, hno 2. When amino acids combine to form proteins or polypeptides a condensation reaction occurs. If acid is added to a solution containing the zwitterion, the carboxylate group captures a hydrogen (h +) ion, and the amino acid becomes positively charged.
Neutral Electrophiles (Compounds Attracted To Regions Of Negative Charge) Also React With Amines;
If acid is added to a solution containing the zwitterion, the carboxylate group captures a hydrogen (h +) ion, and the amino acid becomes positively charged. When amino acids combine to form proteins or polypeptides a condensation reaction occurs. Amines characteristically form salts with acids;
The Amino Acid Side Chain Is Lost As An Aldehyde • The Reaction Of Amino Acids With Ninhydrin Can Detect Amino
Reactions due to side chains 1) ester formation oh containing amino acids e.g. The most important reactions are fair as amino acid chemistry is concerned are the reactions that are utilised in the formation of peptides and proteins. If base is added, ion removal of the h+ ion from the amino group of the zwitterion produces a negatively charged amino acid.
Explain Why Increasing The Concentration Of An Enzyme Does Not Always Increase The Rate Of Reaction.
With the strong mineral acids (e.g., h2so4, hno3, and hcl), the reaction is vigorous. The carboxyl group of one molecule reacts with the amine group of the other molecule. (a) the reaction of 1 o amines (b) the reaction of 2 o amines (c) the reaction of 3 o amines.
The Products Are Hydrochloric Acid And N Methylbenzamide.
When amino acids combine to form proteins or polypeptides a condensation reaction occurs. A hydrogen ion, h+, adds to the nitrogen. Explore four types of amino acid side chains:
What Causes A Protein To Have A Twist, Helix, Or Fold?
Simple chemical tests that are used to detect amino acids take advantage of the reactivity of these functional groups. Suggest two methods of measuring the rate of an enzyme catalysed reaction. Amino acids can be linked by a condensation reaction in which an ―oh is lost from the carboxyl group of.
Popular Posts
Allergy Testing For Chemical Sensitivity
- Get link
- X
- Other Apps
Comments
Post a Comment